Research in the Bronstein group is focused on magnetic nanoparticle based materials in two major thrusts: (i) the development of magnetically recoverable catalysts to obtain value-added chemicals and fuels from biomass and biooil, and (ii) development of magnetic bioprobes for magnetic resonance imaging, hyperthermia and drug delivery.
In general, this work is largely collaborative. They have collaborators in the U.S. and all over the world including Greece, Germany, Russia, and the UK. Collaborations allow Senior scientist Lyuda Bronstein to branch out and to leave her comfort zone, while allowing students to learn collaborative environments, obligations associated with them, and to perform science to which they would typically not be exposed. At Indiana University, Bronstein has ongoing collaborations with Prof. Bogdan Dragnea in the Department of Chemistry and Prof. Aaron Ermel at the IU Medical School.
Interestingly, almost all research in the Bronstein group is performed solely by undergraduate students.
Because of the nature of the Senior Scientist position, Bronstein can only co-chair graduate students when another faculty is willing to share a graduate student. For her entire 18 years at Indiana University, this has happened only three times. The last graduate student jointly shared with Prof. Bogdan Dragnea graduated in 2014 (accepting a position at Caltech) and undergraduate students become the main driving force for scientific progress. This means that undergraduates learn to do everything, every step of the way. Bronstein is available to help, both hands-on in the lab and discussing results in group meeting and personal meetings in the office.
But this environment also creates a unique pressure on the undergraduates: they are not merely helpers, they need to “own” their projects more than many students do in the research groups. This makes them strive for research progress, leading to publications, often resulting in a good recommendation letter for an admittance to a professional or graduate school (although this is not a major motivation). Many of them just get a “bug”: they strive for progress, they want to succeed because it brings them satisfaction. They want to conquer obstacles and solve problems they encountered. They learn to enjoy it!
Methanol synthesis from syngas
One recent project deals with the catalyst development for the methanol synthesis from syngas – one of the important sustainable processes which connects an environmentally significant sequence between biomass or biooil (sources of syngas), methanol, and value-added chemicals such as hydrocarbons or fuels (obtained from methanol). For methanol synthesis, they’ve developed a new family of Ni-, Co-, and Cr-doped Zn-containing magnetic oxide nanoparticles stabilized by two thermally stable polymers, one of which is linear polyphenylquinoxaline (PPQ) and the other is hyperbranched, rigid pyridylphenylene polymer (PPP).

The Zn-containing magnetic oxide nanoparticles (Figure 2) have been synthesized in a one-pot procedure by thermal decomposition of Zn and doping metal acetylacetonates in the reaction solution of preformed magnetite nanoparticles. Using microscopic capabilities of the departmental nanocharacterization facility, they have demonstrated that all three types of metal species (Fe, Zn, and a doping metal) reside within the same nanoparticles, the surface of which is enriched with Zn and a doping metal, while the deeper layers are enriched with Fe, making it preferable for catalysis.
This mixed oxide structure furnished unique catalytic properties in syngas conversion to methanol. Moreover, it was demonstrated that catalytic activities are dependent on the doping metal content and on the stabilizing polymer. The PPP stabilization allows for better access to the catalytic species due to the open and rigid polymer architecture (Figure 2) and most likely optimized distribution of doping species than in the case of PPQ stabilization. Excellent activity and stability of these catalysts make them promising for practical applications. Two undergraduates, Nicholas Baird and Jasper Dittmar, appeared as first and second authors for this project in collaboration from two universities in Russia.
Magnetite-zeolite catalysts
Another project deals with the development of magnetite-zeolite catalysts for the methanol-to-hydrocarbon (MTH) reaction to obtain chemicals and fuels from renewable sources. They have developed hierarchical zeolites (ZSM-5) containing both iron oxide and nickel oxide nanoparticles. Modifying the iron oxide (magnetite, Fe3O4) amounts, they were able to control the catalyst activity and the product distribution in the MTH process. At medium Fe3O4 loading, the major fraction is composed of the C9-C11 hydrocarbons (gasoline fraction). At the higher Fe3O4 loading, the C1-C4 hydrocarbons prevail in the reaction mixture, while at the lowest magnetite loading the major component is C5-C8 hydrocarbons.
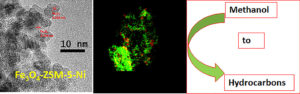
Addition of Ni species to Fe3O4-ZSM-5 leads to the formation of mixed Ni oxides (NiO/Ni2O3) positioned either on top of or next to Fe3O4 nanoparticles (Figure 3). This modification allowed them to significantly improve the catalyst stability due to diminishing coke formation and disordering of the resulting coke. The incorporation of Ni oxide species also leads to a higher catalyst activity and an improved selectivity, making these zeolites highly promising for industrial applications. This work was a joint effort between Bronstein’s group and collaborators from the Tver State Technical University, Russia, and University of Warwick, UK. Former group member, Joshua Mann, is a first author on the published paper.
Since 2014 , her group has published 14 papers and the majority of them had undergraduate students as first authors. There are numerous other projects for undergraduates, but probably the most unique feature of working in the Bronstein lab is how she interacts with these undergraduates when they join her group.
Typically, the first semester is spent in training under supervision of more experienced students in lab. In this semester new students learn procedures pertinent to their future projects. At the same time, Bronstein gives them some relevant literature to read, meeting with them weekly or biweekly (depending on the student availability) to discuss their questions and to enhance their understanding. If students are confused, Bronstein might discuss the same paper 2 – 3 times until they understand it well.
After their training is complete, they begin their own project. Considering that many projects have external collaborators, often students have obligations to provide materials for testing. Hence, there is not time for procrastination. These collaborations help students to be motivated and build team efforts on a number of projects.
Major research progress is done by students during summer research.
Although mentor-mentee teams work well, surprisingly, team work is really difficult skill for students to develop. While these students are friendly, help each other, and socialize well, it is difficult to make them work in teams as equal partners. They often do not talk to each other about their work, and do not discuss their science. Because in the real world team work is an important part of any work place, Bronstein pushes them to learn how they can benefit from a team environment.
Weekly group meetings are crucial. From Prof. Steve Tait’s group experience, they adopted weekly research updates by each student at the beginning of the group meeting. These do not go into much detail (individual meetings are scheduled with each student for detailed discussions), but it stimulates students to think about what they accomplished for the past week. They are quite eager to talk about these accomplishments. It also gives them better awareness about work of their group colleagues. After research updates, students present either their work or a literature paper which is relevant to their research.
During a semester, each student presents their work at least once and receives questions and comments from the group. Students find these experiences invaluable as they have very few opportunities to make presentations in their core courses. If students have to present their work at conferences or at the departmental poster sessions, they first present their draft posters at group meetings and get suggestions from group members on how to improve their posters. This is also a valuable experience for improving their skills in presenting their data.
A major portion of Brontein’s interactions with students is “career counseling”.
Last year thanks to the IU Hutton Honors College Travel Grants, two undergraduates, Joshua Mann and Jasper Dittmar, presented at the fall American Chemical Society (ACS) meeting in Philadelphia. This year two more students, Paige Price and Stanley Bram, will present posters at the fall ACS meeting in Washington, DC. Students feel that it is an awesome experience.

Students are strongly encouraged to participate at national meetings on two conditions: (i) they need to have some results by the abstract submission time and (ii) they need to do research in summer to make sure that there will be more progress by the meeting time.
Major research progress is done by students during summer research. In this respect, the students and Bronstein owes a great deal of gratitude to the Department of Chemistry and the Hutton Honors College for summer scholarships. Without this funding, their research would be completely impeded as the Bronstein group operates without any external funding. Buying chemicals and paying instrumentation fees is a significant part of their budget and during fall and spring semesters their group enjoys some financial support from the Department of Chemistry for those items.
A major portion of Brontein’s interactions with students is “career counseling”. Often, merely doing research helps them to choose between pursuing graduate studies and becoming a health professional. Furthermore, Bronsein takes time to talk to her students about differences in such careers and about their opportunities if they choose one over the other. Although not all of these undergraduate students will become chemistry professionals, she boasts success if they become better researchers when they graduate than when they joined her group.
Students in the Bronstein group are strongly encouraged when they join the group to stay until graduation and to take the capstone course, X499:

Chemical Research Capstone, which includes writing a senior thesis and presenting a poster at the at the annual Chemistry Honors Banquet (Figure 1). The poster session allows them to demonstrate to the department and their peers what they accomplished. Writing a senior thesis affords them a first experience of writing a scientific paper. Both experiences teach them how to focus on the problem to be solved, to talk about the work of others, to write up the experimental part and the most challenging, the Results and Discussion session, where the students have to not only describe their observations but also make sense of them.
Due to a well-structured X499 syllabus, students start working on their thesis early and all spring semester. Bronstein’s job is to teach them to write up scientific results. Normally, it takes several iterations between students and Bronstein to move from the first draft to the final version. Students work hard on their writing, complying with the group requirement of using EndNote for their thesis referencing, and learning an important skill which should be useful in their future careers. In the end, the students’ research experience greatly enhances their education and future career prospects.
Although students’ progress can be slow when they are too busy with classes or other student activities, Bronstein finds it extremely satisfying to be their mentor and to see them grow.